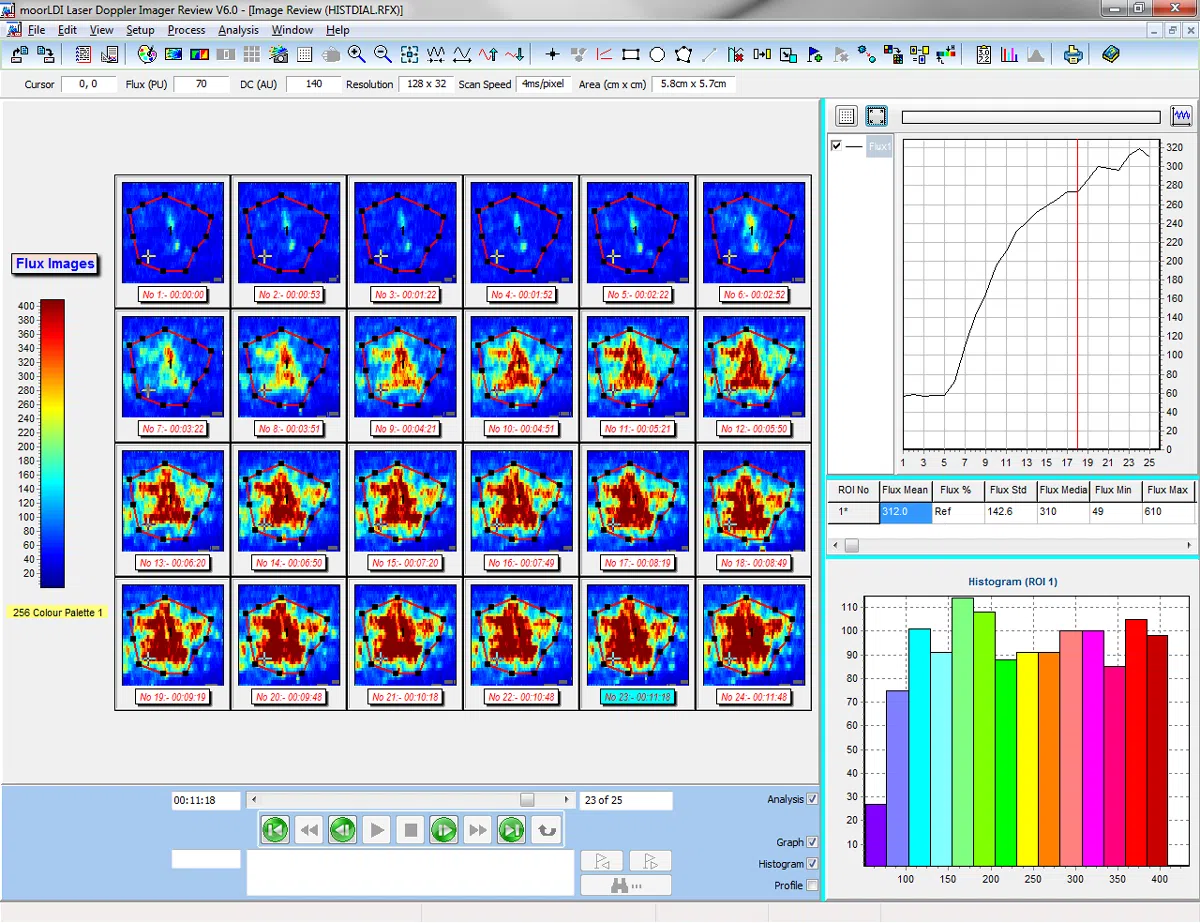
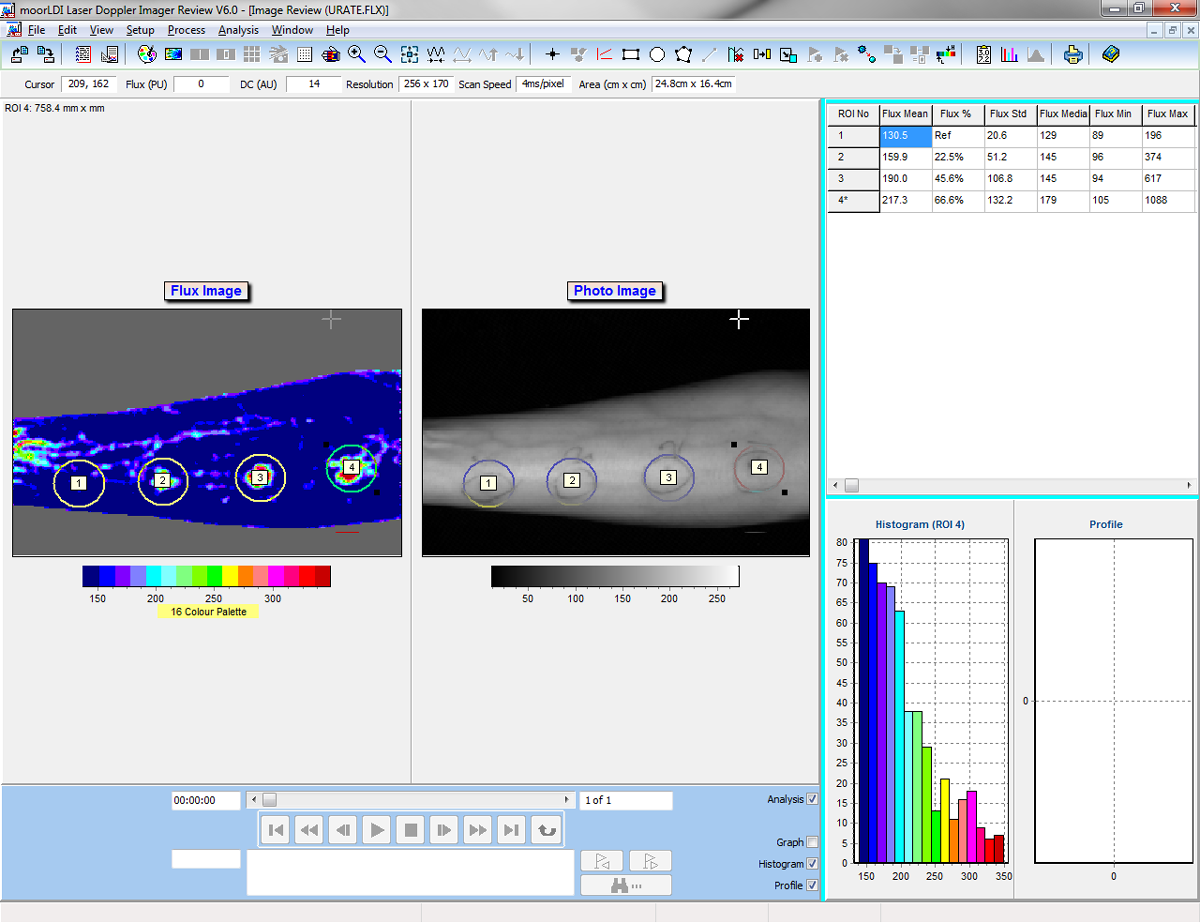
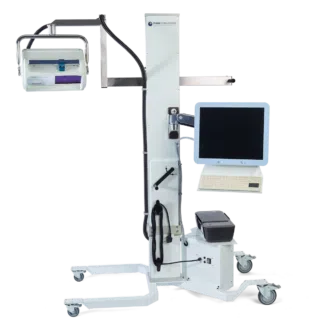
The moorLDI2-IR laser Doppler blood flow imager offers a well proven, high specification solution to your blood flow application for clinical or research application. The system is in routine use in numerous laboratories and clinics globally and employs unique, optical design and signal processing in order to generate the highest resolution and clearest images of its class.
Laser Doppler imaging (LDI) is often compared to laser speckle imaging and whilst there are some similarities, both techniques offer unique advantages. LDI generally offers deeper penetration enabling enhanced visualisation of small vessels below the tissue surface, perfect for angiogenesis modelling or through skull pre clinical cerebral blood flow imaging.
The moorLDI2-IR also allows very large area imaging – up to 50cm x 50cm in one scan. For certain applications these features are critical.
Other features and benefits include;
- Non contact measurement – painless for patient, aids infection control, no chemical tracers or dyes needed.
- Daylight operation – use in most lab, clinic or theatre settings.
- Flexible scan sizes – from a fingertip up to an adult torso.
- High spatial resolution – to catch the finest detail to 100 micron.
- Single and Repeat imaging modes – compare flow from region to region within the same scan and scan the same region repeatedly to assess changes over time.
- Advanced Windows compatible software – to ease setup and scanning. Post Measurement processing functions to make the most of your data.
- Protocol control – set the imager to control flexible tissue heating, pressure cuff control and transdermal drug delivery routines – reproducible, precise and reliable.
- Digital Trigger In/ Out – to synchronise with external devices.
- Digital Signal Processing and High Quality optics – providing the highest sensitivity to changes in blood flow and superb reliability.
- Choice of lasers – to assess from the surface to superficial and deeper tissue beds.
- Choice of stands – for benchtop and clinical/ theatre use.
For the full range of Moor Instruments imagers, please download the imager catalogue here.